Research Labs
To address the absence of research models that can faithfully represent the complexity of IPF, the Beers Lab, along with the 4 UO1 research center teams, have formed a consortium to capitalize upon each center’s unique technologies, models, chemical reagents and expertise to create a platform for understanding the pathogenesis of IPF and foster advancements in the Discovery, Target Identification / Target Validation and Preclinical Testing of new therapies to target IPF.
The overarching goal of the Beers lab is to advance and improve the care of patients with lung disease through research into the causes and to development of novel treatment modalities for lung diseases utilizing an interdisciplinary approach to characterizing the cellular and molecular pathways altered during lung injury and repair processes. Our lab has had a long-standing interest in lung epithelial cell biology in health and disease and we have been world leaders in the fields of surfactant biology and epithelial injury in infectious and inflammatory lung diseases. Based on these discoveries we have pivoted our efforts to understanding the role of the epithelium in fibrotic lung diseases. We recently leveraged the development of multiple mouse strains expressing disease relevant mutations of the surfactant SP-C gene that mimic a rare and devastating relevant fibrotic lung disease called Idiopathic Pulmonary Fibrosis (IPF).
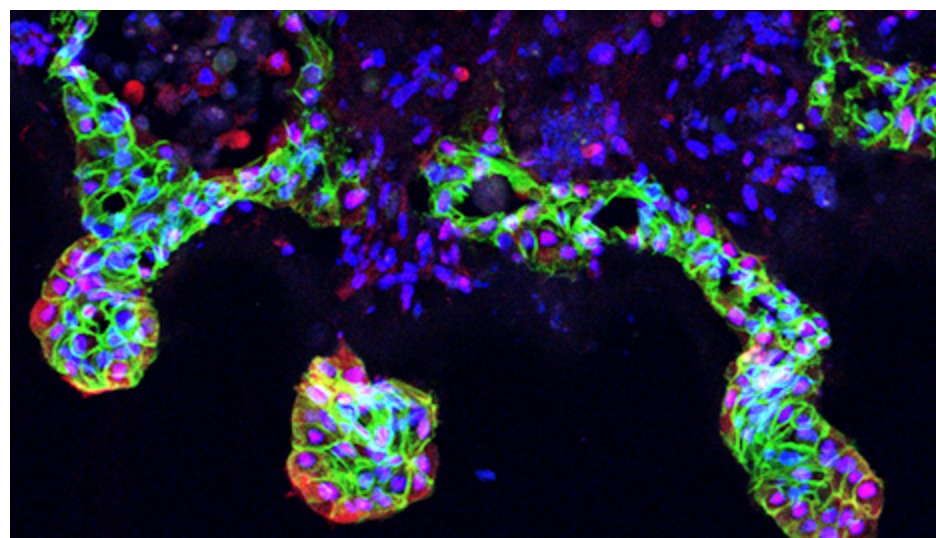
The Gomperts lab have developed a 3D bioengineered human model of IPF that displays progressive fibrosis and closely phenocopies several characteristics associated with IPF. This model is an extension of our 2D model of progressive fibrosis (Vijayaraj et al., Cell Reports). In our 2D model, the fibrosis is seen as aggregates of activated fibroblasts that progressively increase in size and stiffness. This is accompanied by increased secretion of extracellular matrix (ECM), inflammatory cytokines/chemokines and a senescence associated secretory phenotype. This 2D model is representative of known fibrotic molecular and cellular changes and the cellular plasticity that is seen across fibrotic diseases. To make our progressive fibrosis model specific to IPF, we have developed it into a model system that utilizes the lung 3D architecture and specific cell types. Our main goal is to develop a well validated, representative human model of IPF in a dish.
The Kotton lab’s goal is advancing our understanding of lung disease and developmental biology. The group’s approach is founded on the premise that novel treatments for many lung diseases can be realized based on a better understanding of how the lung develops as well as regenerates after lung injury. Together with collaborators in the Kropski and Blackwell Labs from Vanderbilt, the Kotton Lab’s IPF U01 Consortium Project focuses on developing patient-specific pluripotent stem cells to generate novel human lung organoids in vitro that reveal the basic biological mechanisms responsible for IPF or familial pulmonary fibrosis syndromes.
Dr. Rowe’s laboratory specializes in developing new treatments for airway diseases including cystic fibrosis, chronic bronchitis, and other related conditions. He is an expert in the mechanisms underlying mucociliary clearance, developed imaging modalities to measure its effects, and established animal models and other procedures to ascertain pathophysiological processes. These interests led his group to the evaluation of IPF, and its relationship to altered mucin expression using ferrets as a novel animal model of lung injury. He currently directs a translational research program that examines the relationship between mucus biology, clinical phenotype, and therapeutic benefits.
In this project, the Tschumperlin lab will establish robust models in which to study extracellular matrix deposition, the defining characteristic of scarring, and its degradation and resorption. The proposed models will use both primary human fibroblasts and ex vivo tissues cultured from the lungs of individuals with IPF, providing new insight into the failure of scar resorption in IPF, and new targets through which to promote this critical process.